Navigating the intricacies of drug development can seem like delving into a complex labyrinth of scientific, regulatory, and commercial challenges. Yet, understanding this process is crucial for anyone involved in healthcare, from researchers and clinicians to patients and policymakers. In this comprehensive guide, we shed light on the stages, stakeholders, and key considerations that shape the journey from molecule to medicine.
The Drug Development Process: An Overview:
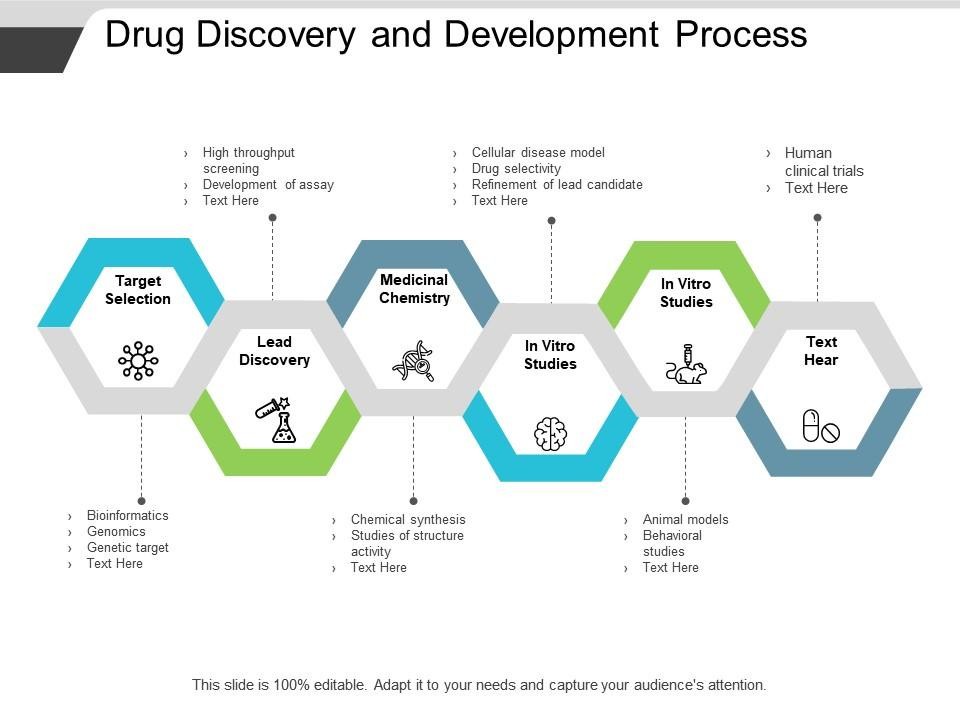
At its core, the drug development process is a meticulously structured journey aimed at transforming a promising scientific concept into a safe, effective, and commercially viable therapy. This journey typically encompasses several distinct stages, each with its own set of objectives, challenges, and milestones. While the specifics may vary depending on factors such as therapeutic area, target population, and regulatory requirements, the overarching framework remains consistent.
Discovery and Preclinical Research: Laying the Foundation:
The journey begins in the laboratory, where researchers embark on the quest to identify novel drug candidates with the potential to address unmet medical needs. This phase, known as drug discovery, involves screening large libraries of compounds, often utilizing high-throughput screening technologies, computational modeling, and structure-activity relationship studies. Once promising candidates are identified, they undergo rigorous preclinical testing to assess safety, efficacy, and pharmacokinetic properties in cellular and animal models. This phase serves as the foundation upon which subsequent development activities are built, providing crucial insights into the therapeutic potential and safety profile of the candidate compound.
Clinical Development: From Bench to Bedside:
Perhaps the most well-known phase of the drug development process, clinical development is where promising candidates are put to the ultimate test: human trials. This phase is divided into three primary stages: Phase I, Phase II, and Phase III clinical trials, each designed to address specific research questions and regulatory requirements. Phase I trials focus on evaluating safety, tolerability, and pharmacokinetics in a small cohort of healthy volunteers, while Phase II trials explore preliminary efficacy and optimal dosing in a larger group of patients with the target disease or condition. Phase III trials represent the pivotal stage, aiming to confirm efficacy, safety, and benefit-risk profile in a large, diverse patient population. Collectively, these trials provide the evidence necessary to support regulatory submissions and eventual market approval.
Regulatory Review and Approval: Navigating the Regulatory Landscape:
Once clinical development is complete, the journey enters the regulatory review and approval phase, where drug developers collaborate closely with regulatory authorities to evaluate the safety, efficacy, and quality of the investigational product. In the United States, this process is overseen by the Food and Drug Administration (FDA), while other regions have their own regulatory agencies, such as the European Medicines Agency (EMA) and the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan. Regulatory submissions typically include comprehensive data packages summarizing preclinical and clinical findings, as well as detailed manufacturing and quality control information. Regulatory agencies conduct rigorous reviews to assess the benefits and risks of the proposed therapy, ultimately deciding whether to grant marketing authorization.
Post-Market Surveillance: Ensuring Continued Safety and Efficacy:
Even after a drug is approved and enters the market, the journey is far from over. Post-market surveillance, also known as pharmacovigilance, plays a critical role in monitoring the ongoing safety and efficacy of approved drugs, detecting and managing adverse events, and identifying emerging risks. Drug developers are required to establish robust pharmacovigilance systems to collect, analyze, and report adverse event data from healthcare professionals, patients, and other sources. Regulatory agencies continuously monitor safety signals and may take regulatory action, such as label updates or product recalls, to protect public health.
Final Thoughts:
The drug development process is a multi-faceted journey that requires collaboration, innovation, and unwavering dedication from a diverse array of stakeholders. From the early stages of discovery and preclinical research to the final stages of regulatory review and post-market surveillance, each phase plays a crucial role in bringing new therapies to patients in need. By demystifying this process and understanding the challenges and opportunities it presents, we can pave the way for continued advancements in healthcare and improve outcomes for patients around the world.

No comments yet